

電化學(xué)沉積是半導(dǎo)體薄膜沉積和微電子制備銅互連的重要制備方法宫补。而在沉積過(guò)程中的成核和生長(zhǎng)對(duì)于半導(dǎo)體薄膜和銅互連的性質(zhì)非常重要檬姥,橢偏儀在位監(jiān)測(cè)提供一種實(shí)時(shí)監(jiān)控薄膜沉積的方法。但是橢偏儀在位監(jiān)測(cè)受到光路設(shè)計(jì)粉怕,實(shí)驗(yàn)裝置健民,固液界面以及光譜解析的影響,構(gòu)建其監(jiān)測(cè)系統(tǒng)是一個(gè)挑戰(zhàn)贫贝。
展示全部 
橢偏儀在位表征電化學(xué)沉積的系統(tǒng)搭建(三)-應(yīng)用案例
1.3應(yīng)用案例
橢偏儀在位監(jiān)測(cè)已經(jīng)廣泛應(yīng)用于薄膜生長(zhǎng)秉犹、顆粒和生物大分子的吸附等領(lǐng)域蛉谜。下面介紹一下橢偏儀在位監(jiān)測(cè)在薄膜生長(zhǎng)和顆粒方面的案例。
1.3.1薄膜生長(zhǎng)
橢偏儀對(duì)厚度的無(wú)損測(cè)量使其可實(shí)現(xiàn)薄膜生長(zhǎng)的實(shí)時(shí)監(jiān)控崇堵。而不同時(shí)間生長(zhǎng)時(shí)間其薄膜的性質(zhì)及厚度不同型诚,這樣需要構(gòu)建不同厚度的多層膜結(jié)構(gòu),從而實(shí)現(xiàn)在位監(jiān)控筑辨,得到薄膜生長(zhǎng)厚度隨時(shí)間的變化信息俺驶。比如F.N.Dultsev等采用橢偏儀研究了沉積在硅表面的鈦基體氮化機(jī)理、Yuki Ishikawa等采用原位橢偏儀研究了離子液體薄膜的玻璃化轉(zhuǎn)變行為棍辕,Meng Yuan等提出了一種簡(jiǎn)便暮现、無(wú)損傷的在位橢圓偏振法來(lái)監(jiān)測(cè)CsPbI3薄膜在室溫至340℃熱變色過(guò)程中三個(gè)顯著相變的光學(xué)性質(zhì)演變等。
除了上述橢偏儀常規(guī)薄膜的研究以外楚昭,橢偏儀還用于自組裝單層膜(SAMs)厚度的研究栖袋。自早期實(shí)驗(yàn)以來(lái),橢圓偏振譜已被廣泛應(yīng)用于自組裝單層膜的厚度測(cè)定抚太。利用橢偏儀測(cè)量得到SAMs表面光譜特性塘幅,可以得到SAMs的光學(xué)性質(zhì)以及形態(tài)結(jié)構(gòu),如界面形態(tài)等尿贫。隨著自組裝單層膜(SAMs)監(jiān)測(cè)表征技術(shù)的發(fā)展电媳,紅外橢圓偏振光譜(IRSE)作為表征納米結(jié)構(gòu)的一種強(qiáng)有力的工具,特別是自組裝單分子膜(SAMs)的表征上庆亡,已得到極大的發(fā)展匾乓。與傳統(tǒng)的傅里葉變換紅外反射吸收光譜(FT-IRRAS)相比,IRSE在測(cè)定高反射率波長(zhǎng)區(qū)域內(nèi)的介電函數(shù)(低至單分子層厚度)方面具有優(yōu)勢(shì)又谋。另外拼缝,IRSE表征比FT-IRRAS表征有更多的實(shí)驗(yàn)參數(shù),可以獲取薄膜樣品的更多信息彰亥。
圖1-3為利用橢偏儀在位監(jiān)控微晶mc-Si:H薄膜在ZnO襯底的生長(zhǎng)咧七。生長(zhǎng)模型為島狀生長(zhǎng),因此在生長(zhǎng)過(guò)程中任斋,表面較為粗糙继阻,通過(guò)模型構(gòu)建可以獲取薄膜表面粗糙度隨時(shí)間演變和生長(zhǎng)速率和生長(zhǎng)模式。
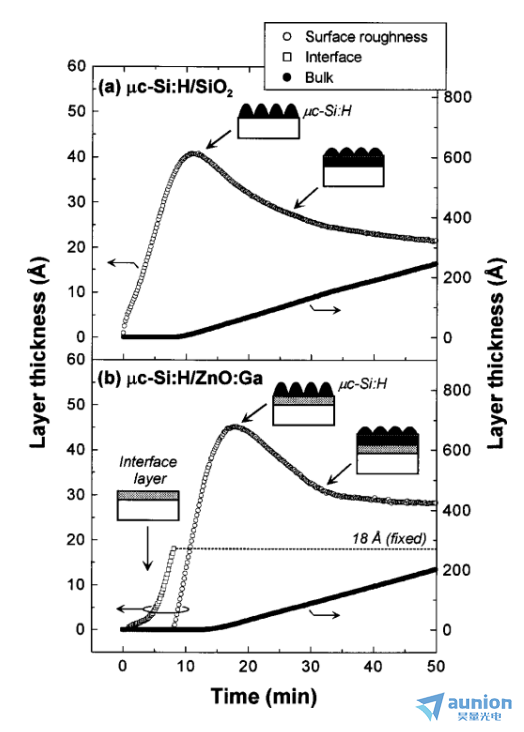
圖1-3薄膜生長(zhǎng)過(guò)程中表面的粗糙度隨著時(shí)間的演變
1.3.2監(jiān)測(cè)顆粒吸附
對(duì)于顆寥示恚或者大分子層的吸附穴翩,橢偏儀可以檢測(cè)到其光學(xué)常數(shù)的變化,并且利用有效介質(zhì)模型提取顆粒的覆蓋率信息等锦积。橢偏儀被廣泛應(yīng)用于生物大分子特別是蛋白質(zhì)等的吸附研究。Woo-KulLee等在2003年采用在位橢偏儀監(jiān)測(cè)蛋清溶菌酶吸附動(dòng)力學(xué)數(shù)據(jù)歉嗓,從而建立了蛋清溶菌酶對(duì)親水二氧化硅吸附動(dòng)力學(xué)的模擬模型丰介。如圖1-4所示,Katerina Stamataki使用橢偏儀(EW-CRDE)采用740nm探測(cè)激光束監(jiān)測(cè)吸附的羅丹明800的吸收和相移Δ以及聚四氟乙烯懸浮液在熔融石英棱鏡表面沉降過(guò)程中的吸收和相移Δ。結(jié)果表明哮幢,橢偏儀為Δ的測(cè)量提供了一種靈敏的方法带膀,Δ的精度約為10-4度。
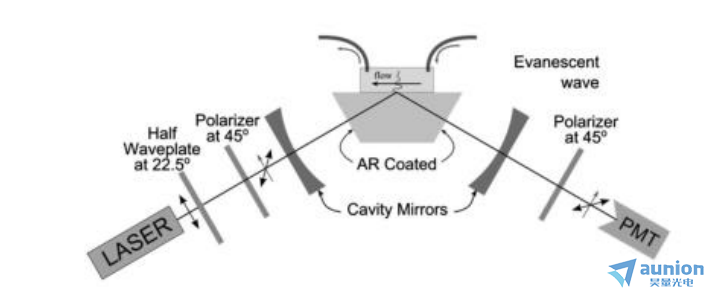
圖1-4用于研究在棱鏡界面處的氣體或液體樣品的實(shí)驗(yàn)裝置
圖1-5是Diana Viegas等用橢偏譜法研究核殼金屬有機(jī)納米粒子吸附在基底上的測(cè)試示意圖橙垢。其采用Bobbert-Vlieger模型計(jì)算核殼粒子在基底上的光散射垛叨,數(shù)值計(jì)算預(yù)測(cè)金屬有機(jī)粒子對(duì)應(yīng)的橢偏參數(shù)Δ和ψ。理論上在裸露的金納米顆粒的極限情況下柜某,Bobbert-Vlieger模型的預(yù)測(cè)與常用的Maxwell-Garnett有效介質(zhì)近似的預(yù)測(cè)一致嗽元。Bobbert-Vlieger模型的優(yōu)點(diǎn)包括它依賴(lài)于麥克斯韋方程組的精確解,以及可以模擬比EMA模型更復(fù)雜的納米結(jié)構(gòu)體系喂击。理論和實(shí)驗(yàn)上都發(fā)現(xiàn)剂癌,在真實(shí)的實(shí)驗(yàn)條件下,可以檢測(cè)到與納米顆粒表面生物功能化和生物認(rèn)知事件相關(guān)的橢偏參數(shù)的變化翰绊。結(jié)果還表明佩谷,這種方法可擴(kuò)展到更復(fù)雜參數(shù)的測(cè)量,例如生物有機(jī)殼的水合程度监嗜,甚至可能擴(kuò)展到溶液中生物功能化納米顆粒的測(cè)量谐檀。
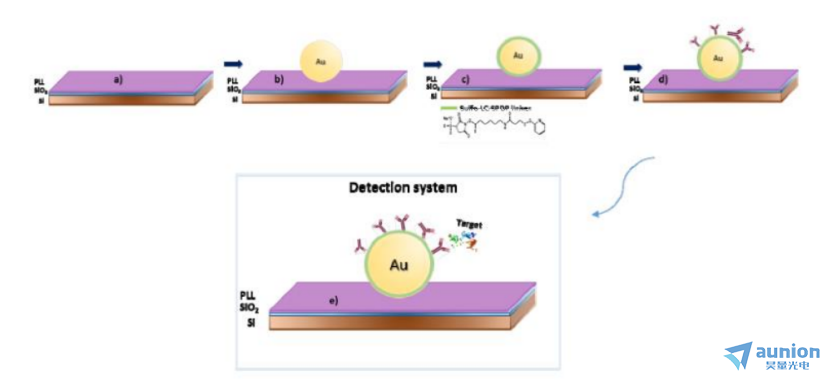
圖1-5 Au納米顆粒探測(cè)有機(jī)分子的示意圖
由此可見(jiàn)橢偏譜通過(guò)建模可以獲取薄膜裁奇、納米顆粒的光學(xué)常數(shù)和生長(zhǎng)過(guò)程信息桐猬。
1.3.3橢偏儀應(yīng)用于電化學(xué)沉積監(jiān)測(cè)
橢偏儀可獲取固-液界面和固-氣界面信息。橢偏儀可用于探測(cè)蛋白分子在固體-液體界面的物理和化學(xué)過(guò)程框喳,獲取蛋白質(zhì)分子的振動(dòng)能量變化课幕。利用橢偏儀可觀察溶液中Mg合金氧化生成MgO的過(guò)程及GaAs的腐蝕過(guò)程。此外五垮,橢偏儀還被應(yīng)用于有機(jī)溶液的介電常數(shù)測(cè)試乍惊,可實(shí)現(xiàn)對(duì)過(guò)濾膜中的固液或固氣界面的生成物的實(shí)時(shí)監(jiān)控。因此橢偏儀表征是固-液界面的重要表征方法之一放仗。利用橢偏儀在位監(jiān)測(cè)Au襯底上Bi2Te3生長(zhǎng)的橢偏參數(shù)并解構(gòu)出電化學(xué)沉積過(guò)程润绎。橢偏儀也用于研究銅互連工藝中有機(jī)高分子PEG與C1-的相互作用對(duì)于Cu沉積過(guò)程的影響。因此在位橢偏儀可直接監(jiān)控電化學(xué)沉積過(guò)程中的厚度和成分變化及相應(yīng)的生長(zhǎng)過(guò)程诞挨。
了解更多橢偏儀詳情棉磨,請(qǐng)?jiān)L問(wèn)上海昊量光電的官方網(wǎng)頁(yè):
http://www.wjjzl.com/three-level-56.html
更多詳情請(qǐng)聯(lián)系昊量光電/歡迎直接聯(lián)系昊量光電
關(guān)于昊量光電:
上海昊量光電設(shè)備有限公司是光電產(chǎn)品專(zhuān)業(yè)代理商,產(chǎn)品包括各類(lèi)激光器祭衩、光電調(diào)制器壹瘟、光學(xué)測(cè)量設(shè)備、光學(xué)元件等银室,涉及應(yīng)用涵蓋了材料加工涂佃、光通訊励翼、生物醫(yī)療、科學(xué)研究辜荠、國(guó)防汽抚、量子光學(xué)、生物顯微伯病、物聯(lián)傳感造烁、激光制造等;可為客戶(hù)提供完整的設(shè)備安裝午笛,培訓(xùn)惭蟋,硬件開(kāi)發(fā),軟件開(kāi)發(fā)季研,系統(tǒng)集成等服務(wù)敞葛。
您可以通過(guò)我們昊量光電的官方網(wǎng)站www.wjjzl.com了解更多的產(chǎn)品信息,或直接來(lái)電咨詢(xún)4006-888-532与涡。
相關(guān)文獻(xiàn):
[1] WONG H S P, FRANK D J, SOLOMON P M et al. Nanoscale cmos[J]. Proceedings of the IEEE, 1999, 87(4): 537-570.
[2] LOSURDO M, HINGERL K. ellipsometry at the nanoscale[M]. Springer Heidelberg New York Dordrecht London. 2013.
[3] DYRE J C. Universal low-temperature ac conductivity of macroscopically disordered nonmetals[J]. Physical Review B, 1993, 48(17): 12511-12526. DOI:10.1103/PhysRevB.48.12511.
[4] CHEN S, KüHNE P, STANISHEV V et al. On the anomalous optical conductivity dISPersion of electrically conducting polymers: Ultra-wide spectral range ellipsometry combined with a Drude-Lorentz model[J]. Journal of Materials Chemistry C, 2019, 7(15): 4350-4362.
[5] 陳籃惹谐,周巖. 膜厚度測(cè)量的橢偏儀法原理分析[J]. 大學(xué)物理實(shí)驗(yàn), 1999, 12(3): 10-13.
[6] ZAPIEN J A, COLLINS R W, MESSIER R. Multichannel ellipsometer for real time spectroscopy of thin film deposition from 1.5 to 6.5 eV[J]. Review of Scientific Instruments, 2000, 71(9): 3451-3460.
[7] DULTSEV F N, KOLOSOVSKY E A. Application of ellipsometry to control the plasmachemical synthesis of thin TiONx layers[J]. Advances in Condensed Matter Physics, 2015, 2015: 1-8.
[8] DULTSEV F N, KOLOSOVSKY E A. Application of ellipsometry to control the plasmachemical synthesis of thin TiONx layers[J]. Advances in Condensed Matter Physics, 2015, 2015: 1-8.
[9] YUAN M, YUAN L, HU Z et al. In Situ Spectroscopic Ellipsometry for Thermochromic CsPbI3 Phase Evolution Portfolio[J]. Journal of Physical Chemistry C, 2020, 124(14): 8008-8014.
[10] 焦楊景.橢偏儀在位表征電化學(xué)沉積的系統(tǒng)搭建.云南大學(xué)說(shuō)是論文,2022.
[11] CANEPA M, MAIDECCHI G, TOCCAFONDI C et al. Spectroscopic ellipsometry of self assembLED monolayers: Interface effects. the case of phenyl selenide SAMs on gold[J]. Physical Chemistry Chemical Physics, 2013, 15(27): 11559-11565. DOI:10.1039/c3cp51304a.
[12] FUJIWARA H, KONDO M, MATSUDA A. Interface-layer formation in microcrystalline Si:H growth on ZnO substrates studied by real-time spectroscopic ellipsometry and infrared spectroscopy[J]. Journal of Applied Physics, 2003, 93(5): 2400-2409.
[13] FUJIWARA H, TOYOSHIMA Y, KONDO M et al. Interface-layer formation mechanism in (formula presented) thin-film growth studied by real-time spectroscopic ellipsometry and infrared spectroscopy[J]. Physical Review B - Condensed Matter and Materials Physics, 1999, 60(19): 13598-13604.
[14] LEE W K, KO J S. Kinetic model for the simulation of hen egg white lysozyme adsorption at solid/water interface[J]. Korean Journal of Chemical Engineering, 2003, 20(3): 549-553.
[15] STAMATAKI K, PAPADAKIS V, EVEREST M A et al. Monitoring adsorption and sedimentation using evanescent-wave cavity ringdown ellipsometry[J]. Applied Optics, 2013, 52(5): 1086-1093.
[16] VIEGAS D, FERNANDES E, QUEIRóS R et al. Adapting Bobbert-Vlieger model to spectroscopic ellipsometry of gold nanoparticles with bio-organic shells[J]. Biomedical Optics Express, 2017, 8(8): 3538.
[17] ARWIN H. Application of ellipsometry techniques to biological materials[J]. Thin Solid Films, 2011, 519(9): 2589-2592.
[18] ZIMMER A, VEYS-RENAUX D, BROCH L et al. In situ spectroelectrochemical ellipsometry using super continuum white laser: Study of the anodization of magnesium alloy [J]. Journal of Vacuum Science & Technology B, 2019, 37(6): 062911.
[19] ZANGOOIE S, BJORKLUND R, ARWIN H. Water Interaction with Thermally Oxidized Porous Silicon Layers[J]. Journal of The Electrochemical Society, 1997, 144(11): 4027-4035.
[20] KYUNG Y B, LEE S, OH H et al. Determination of the optical functions of various liquids by rotating compensator multichannel spectroscopic ellipsometry[J]. Bulletin of the Korean Chemical Society, 2005, 26(6): 947-951.
[21] OGIEGLO W, VAN DER WERF H, TEMPELMAN K et al. Erratum to ― n-Hexane induced swelling of thin PDMS films under non-equilibrium nanofiltration permeation conditions, resolved by spectroscopic ellipsometry‖ [J. Membr. Sci. 431 (2013), 233-243][J]. Journal of Membrane Science, 2013, 437: 312..
[22] BROCH L, JOHANN L, STEIN N et al. Real time in situ ellipsometric and gravimetric monitoring for electrochemistry experiments[J]. Review of Scientific Instruments, 2007, 78(6).
[23] BISIO F, PRATO M, BARBORINI E et al. Interaction of alkanethiols with nanoporous cluster-assembled Au films[J]. Langmuir, 2011, 27(13): 8371-8376.
[24] 李廣立. 氧化亞銅薄膜的制備及其光電性能研究[D]. 西南交通大學(xué), 2016.
[25] 董金礦. 氧化亞銅薄膜的制備及其光催化性能的研究[D]. 安徽建筑大學(xué), 2014.
[26] 張楨. 氧化亞銅薄膜的電化學(xué)制備及其光催化和光電性能的研究[D]. 上海交通大學(xué)材料科 學(xué)與工程學(xué)院, 2013.
[27] DISSERTATION M. Cellulose Derivative and Lanthanide Complex Thin Film Cellulose Derivative and Lanthanide Complex Thin Film[J]. 2017.
[28] NIE J, YU X, HU D et al. Preparation and Properties of Cu2O/TiO2 heterojunction Nanocomposite for Rhodamine B Degradation under visible light[J]. ChemistrySelect, 2020, 5(27): 8118-8128.
[29] STRASSER P, GLIECH M, KUEHL S et al. Electrochemical processes on solid shaped nanoparticles with defined facets[J]. Chemical Society Reviews, 2018, 47(3): 715-735.
[30] XU Z, CHEN Y, ZHANG Z et al. Progress of research on underpotential deposition——I. Theory of underpotential deposition[J]. Wuli Huaxue Xuebao/ Acta Physico - Chimica Sinica, 2015, 31(7): 1219-1230.
[31] PANGAROV n. Thermodynamics of electrochemical phase formation and underpotential metal deposition[J]. Electrochimica Acta, 1983, 28(6): 763-775.
[32] KAYASTH S. ELECTRODEPOSITION STUDIES OF RARE EARTHS[J]. Methods in Geochemistry and Geophysics, 1972, 6(C): 5-13.
[33] KONDO T, TAKAKUSAGI S, UOSAKI K. Stability of underpotentially deposited Ag layers on a Au(1 1 1) surface studied by surface X-ray scattering[J]. Electrochemistry Communications, 2009, 11(4): 804-807.
[34] GASPAROTTO L H S, BORISENKO N, BOCCHI N et al. In situ STM investigation of the lithium underpotential deposition on Au(111) in the air- and water-stable ionic liquid 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide[J]. Physical Chemistry Chemical Physics, 2009, 11(47): 11140-11145.
[35] SARABIA F J, CLIMENT V, FELIU J M. Underpotential deposition of Nickel on platinum single crystal electrodes[J]. Journal of Electroanalytical Chemistry, 2018, 819(V): 391-400.
[36] BARD A J, FAULKNER L R, SWAIN E et al. Fundamentals and Applications[M]. John Wiley & Sons, Inc, 2001.
[37] SCHWEINER F, MAIN J, FELDMAIER M et al. Impact of the valence band structure of Cu2O on excitonic spectra[J]. Physical Review B, 2016, 93(19): 1-16.
[38] XIONG L, HUANG S, YANG X et al. P-Type and n-type Cu2O semiconductor thin films: Controllable preparation by simple solvothermal method and photoelectrochemical properties[J]. Electrochimica Acta, 2011, 56(6): 2735-2739.
[39] KAZIMIERCZUK T, FR?HLICH D, SCHEEL S et al. Giant Rydberg excitons in the copper oxide Cu2O[J]. Nature, 2014, 514(7522): 343-347.
[40] RAEBIGER H, LANY S, ZUNGER A. Origins of the p-type nature and cation deficiency in Cu2 O and related materials[J]. Physical Review B - Condensed Matter and Materials Physics, 2007, 76(4): 1-5.
[41] 舒云. Cu2O薄膜的電化學(xué)制備及其光電化學(xué)性能的研究[D]. 云南大學(xué)物理與天文學(xué)院,2019.
展示全部 